Avogadro Number Analogies
Avogadro is an advanced molecule editor and visualizer designed for cross-platform use in computational chemistry, molecular modeling, bioinformatics, materials science, and related areas. It offers flexible high quality rendering and a powerful plugin architecture. Avogadro’s Law Examples in Real Life Avogadro’s law tells about the relationship between the volume of a gas and the number of molecules possessed by it. It was formulated by an Italian scientist Amedeo Avogadro in the year 1811. The number of molecules in 1 mole is known as Avogadro’s Number, and is huge (6.02x10 23)! Note, we are not restricted to these conditions. For example, we could change the volume for these gases to be higher or lower and change the pressure.
Avogado's Number is so large many students have trouble comprehending its size. Consequently, a small sidelight of chemistry instruction has developed for writing analogies to help express how large this number actually is.
Before looking over the following examples, here's a nice YouTube video about the mole and Avogadro's Number. Have fun and please come back to the ChemTeam when you're done exploring.
1) Avogadro's Number compared to the Population of the Earth:
We will take the population of the earth to be six billion (6 x 109 people). We compare to Avogadro's Number like this:
6.022 x 1023 divided by 6 x 109 = approx. 1 x 1014
In other words, it would take about 100 trillion Earth populations to sum up to Avogadro's number.
If we were to take a value of 7 billion (approximate population in 2012), it would take about 86 trillion Earth populations to sum up to Avogadro's Number.
2) Avogadro's Number as a Balancing Act:
At the very moment of the Big Bang, you began putting H atoms on a balance and now, 19 billion years later, the balance has reached 1.008 grams. Since you know this to be Avogadro Number of atoms, you stop and decide to calculate how many atoms per second you had to have placed.
1.9 x 1010 yrs x 365.25 days/yr x 24 hrs/day x 3600 sec/hr = 6.0 x 1017 seconds to reach one mole6.022 x 1023 atoms/mole divided by 6.0 x 1017 seconds/mole = approx. 1 x 106 atoms/second
So, after placing one million H atoms on a balance every second for 19 billion years, you get Avogadro Number of H atoms (approximately).
3) Avogadro's Number in Outer Space:
If all the matter in the universe were spread evenly throughout the entire universe, there would be approx. 1 x 10¯6 nucleons per cm3. We could do several things with that. For example:
a) What volume (in cm3) of space would hold Avogadro Number of nucleons?6.022 x 1023 nucleons/mole divided by 1 x 10¯6 nucleons/cm3 = 6.022 x 1029 cm3/moleb) How many Earths would equal this volume of space (take Earth's radius to be 6380 km)?
4) Avogadro Number of Coins:
Take a common coin of your country and stack up 30 of them. Measure the height in cm and divide by 30. You now have the average height of one coin in centimeters.
a) How high in cm is a stack of Avogadro Number of that coin?
b) How many light years is this? (Light travels 3.00 x 108 km per second)
c) How many 'round-trips' is this to the moon? (Go there and back = one round-trip. The Earth-Moon distance (measured center-to-center is a bit more than 384,000 km.)
Another way to express this type of problem: If you placed one mole of pills (coins, etc.) with a diameter of 1.00 cm side by side, how many trips around the Sun's equator can you make?
Solution:
1) Convert diameter of Sun from km to cm:
(1.392 x 106 km x (105 cm / 1 km) = 1.392 x 1011 cm
cmAvogadro's Principle Example
I looked up the diameter of the Sun online.
2) Calculate circumference of sun:c = πdc = (3.14159) (1.392 x 1011 cm)
c = 4.3731 x 1011 cm
3) Calculate trips around the Sun:
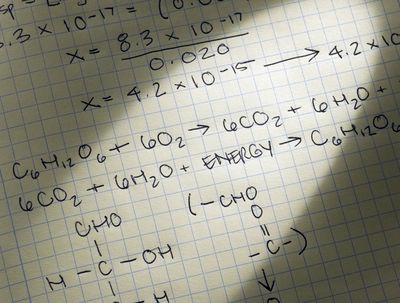 Since each pill = 1.00 cm, one mole of them covers 6.022 x 1023 cm
Since each pill = 1.00 cm, one mole of them covers 6.022 x 1023 cm6.022 x 1023 cm / 4.3731 x 1011 cm
1.377 x 1012 times around the Sun.
5) Avogadro Number of Pieces of Paper:
If you had a mole of sheets of paper stacked on top of each other, how many round trips to the Moon could you make? (Hint: a stack of 100 sheets of ordinary printer paper is about 1.0 cm.)
6) The area of the ChemTeam's home state of California is 403932.8 km2. Suppose you had 6.022 x 1023 sheets of paper, each with dimensions 30 cm x 30 cm. (a) How many times could you cover California completely with paper? (b) Suppose each sheet of paper is 1 mm thick. How high would the paper be stacked?
7) If you drove 6.022 x1023 days at a speed of 100 km/h, how far would you travel?
8) If you spent 6.022 x 1023 dollars at an average rate of 1.00 dollar/s, how long in years would the money last? (Assume that every year has 365 days.)
The Extensions Menu is a catalog of computational plugins equipped with Avogadro. These plugins can interact with molecules, generate input file dialogs for quantum codes, and create molecule property dialogs.
Animation
Selecting “Animation” will open the animate trajectory dialog box shown below. From here you can load a file, view and edit the animation, as well as save the file in a PC compatible format.
Optimize Geometry
“Optimize Geometry” provides a quick, realistic rendition of a molecule using molecular mechanics.
Molecular Mechanics
“Molecular Mechanics” allows you to edit the geometry optimization of a molecule, so that it best suits your purposes.
Setup Force Field…
A dialog box will open when “Setup Force Field…” is selected. This dialog box provides you with the ability to choose the type of force field, and algorithm that can best optimize your molecular parameters, and preferences.
Calculate Energy
“Calculate Energy” determines the amount of energy per the amount of material (kJ/mol), and displays this number in a pop up dialog box.
Conformer Search
“Conformer Search” is a way to easily search for conformers within a molecule (dialog box shown below). A more detailed outline on how to perform a conformer search is found in the “Optimizing Geometry” section of this manual. Avogadro only renders staggered conformations, and does not calculate ring conformers.
Constraints
“Constraints” is a way to ensure atom stability in various selections (dialog box depicted below). The constraints that can be applied to a molecule include Ignore Atom, Fix Atom, Fix Atom X, Fix Atom Y, Fix Atom Z, Distance, Angle, and Torsion Angle. A detailed outline on how to use the constraints feature is found in the “Optimizing Geometry” section of this manual.
Ignore Selection
“Ignore Selection” allows you to select a specific part of a molecule to omit during a geometry optimization.

Fix Selected Atoms
“Fix Selected Atoms” also allows you to set a certain part of a molecule to fix during optimization.
Avogadro Extensions–Plugins
Avogadro's Number Example
Avogadro provides you with the ability to interface your molecules with other dialog based plugins. These extensions interact with a molecule to provide further molecular information, and additional computation abilities. These plugins include but aren’t limited to GAMESS, Abinit, Dalton, GAMESS-UK, Gaussian, MOLPRO, MOPAC, NWChem, PSI4, Q-Chem, and LAMMPS.
General “How To” for Plugins
Avogadro (as you will see below) can be used to display molecular orbitals, QTAIM, spectra, as well as create surfaces. However, many of these features can not be used to their full potential without first running one of the plugins listed in the section above. Gaussian is one of the most common plugins used, due to it’s wide range of basis sets/functions.
Running Gaussian
After selecting “Gaussian” from the Extensions menu, the dialog box depicted below will appear. You can edit the dialog box and add specific keywords to utilize these features in Avogadro. For example, typing “freq” in the dialog box will compute force constants and vibrational frequencies. More information on keywords for Gaussian can be found at the Gaussian website (http://www.gaussian.com/g_tech/g_ur/l_keywords09.htm). Then clicking generate will let you save the file to your computer, so you can run the file in external software.
Once the file has been run through the external software, you will have a .g03 or .g09 file that will open the keyword selection in a toolbar on the right hand side of the screen. “Freq” will open the vibrations toolbar shown below.
Molecular Orbitals
The “Molecular Orbitals” selection will display the molecular orbitals for orbitals with full status bars. The quality of the orbitals can be adjusted an reconfigured if need be. This feature only works by running gaussian extension files (.fchk, .g03, .g09, etc.).
QTAIM (Quantum Theory of Atoms in Molecules)
QTAIM displays the implicit bonding that is theorized to take place between the hydrogens of organic crystals (the implicit bonding is conveyed through dots). This display type is utilized by importing a .wfn file from the “QTAIM”, “Molecular Graph” selection under the “Extensions” menu. Selecting “Molecular Graph with Lone Pairs” or “Atomic Charge” will provide concurrent information about the molecule. More information can be found on this process in the Tutorial section of this manual.
Spectra…

Clicking on “Spectra…” will create a spectra visualization of a .g03, or .g09 file that has been run with the keyword “freq”. A spectral visualization can also be created through the vibrations toolbar by selecting “Show Spectra…”.
Create Surfaces…
“Create Surfaces…” allows you to view the Van der Waals, Electrostatic Potential, Electron Density, and Molecular Orbital Surfaces. The surface type options for viewing depend on what type of calculations have previously been run on the molecule. The type of file you open/create allows for more or less surface viewing options (generally discussed under Avogadro Extensions–Plugins). This feature also allows you to edit the color, resolution, and iso value to further enhance your surface.
